Non-Equilibrium Plasma for PFAS Treatment in Water

Researchers are refining non-traditional approaches to removing “forever chemicals” from water.
With growing concern about PFAS (per- and polyfluoroalkyl substances) contamination in drinking water, innovative approaches to water treatment are essential. Researchers have recently investigated using non-equilibrium plasma systems to mitigate PFAS, such as PFOA and PFOS, in contaminated water.
You can also read: What’s All The Fuss About PFAS?
Approaches to PFAS Degradation
Traditionally, water treatments mineralize (converted to carbon oxides and HF) PFAS using thermal techniques. These techniques include incineration, thermal desorption, pyrolysis and gasification, smoldering combustion, and hydrothermal liquefaction. Researchers are investigating alternatives because these approaches can be energy-intensive and may create harmful byproducts. One promising solution to these challenges is non-equilibrium plasma treatment.
What is Non-Equilibrium Plasma?
Non-equilibrium plasma features electron temperatures that are much hotter than the rest of the partially ionized gas. Various factors can control the reactive environment that non-equilibrium plasma creates. Non-equilibrium plasma systems are configurable for multiple applications, including PFAS treatment. In previous studies using a variety of system configurations, complete mineralization of PFAS remains challenging to achieve.
Tailoring the System
To better optimize this method, researchers investigated the amount of energy needed to mineralize PFAS. With this information, it is possible to tailor systems for PFAS in water to be more efficient. The researchers created a model of PFOA defluorination as a function of enthalpy. To validate the model, researchers treated PFOA and PFOS-contaminated water using a gliding arc plasmatron (GAP) system. Then, to estimate the activation energy of reactive species associated with PFAS degradation, researchers conducted a preliminary kinetic analysis.
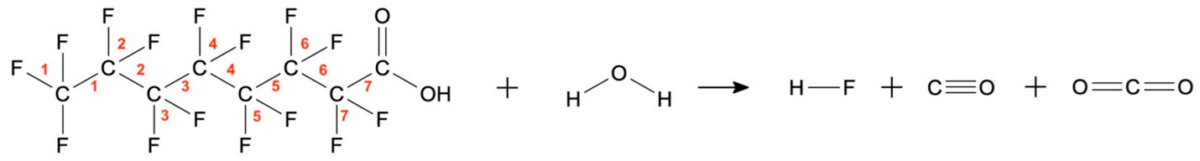
PFOA mineralization in water. Courtesy of Plasma-Assisted Abatement of Per- and Polyfluoroalkyl Substances (PFAS): Thermodynamic Analysis and Validation in Gliding Arc Discharge.
A Lower Temperature Requirement
The model’s results demonstrated that the mineralization process requires a temperature of about 195 °C to occur. This temperature is lower than the requirement for the traditional thermal treatment of PFAS-contaminated water. For example, the incineration of PFAS-contaminated matrices requires temperatures that are greater than 900 °C. The model theoretically confirms the direct relationship of enthalpy and PFOA defluorination. Going forward, this information can aid in assessing various treatment methods.
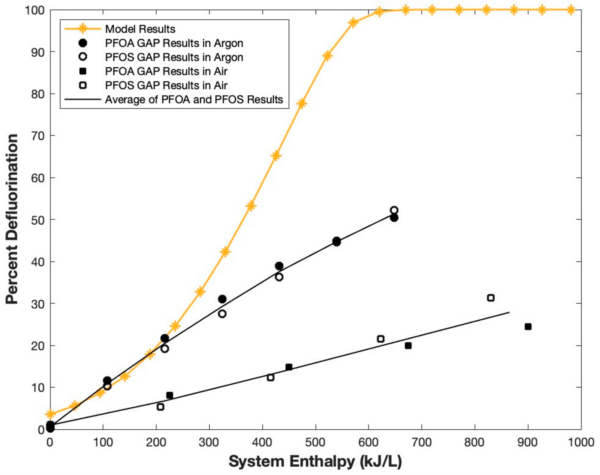
Modeling PFOA defluorination as a function of system enthalpy. Courtesy of Plasma-Assisted Abatement of Per- and Polyfluoroalkyl Substances (PFAS): Thermodynamic Analysis and
Continued Research
Since this is a theoretical model, further research will be necessary to monitor the behavior of PFAS. The model does not consider reactive species present in non-equilibrium plasma systems or transformation products in the reaction system. Additionally, the model does not consider several properties of PFAS that may impact their mineralization, such as surfactant properties. Nevertheless, these findings pose a promising approach towards the mineralization of PFAS in water matrices. Traditional methods will need to be reevaluated as the need for energy efficient and scalable PFAS treatment increases globally. The optimization of non-equilibrium plasma systems is a promising step towards the goal of effective cleanup.
