Durable Plastics That Biodegrade in Oceans

A team of researchers at the RIKEN Center for Emergent Matter Science has created an innovative plastic designed to combat microplastic pollution.
Researchers led by Takuzo Aida at the RIKEN Center for Emergent Matter Science (CEMS) have developed a groundbreaking plastic that promises to tackle microplastic pollution.
You can also read: Revolutionizing Materials: The Rise of Supramolecular Polymers.
Unlike traditional plastics, this innovative material is strong, biodegradable, and designed to break down in seawater. Published on November 22 in Science, this discovery represents a major step forward in creating sustainable alternatives to conventional plastics.
Addressing the Microplastic Crisis
Microplastics, tiny particles less than 5 mm in size, pose a significant threat to marine ecosystems and human health by entering the food chain. Current biodegradable plastics, such as PLA, fail to degrade in ocean environments due to their water-insoluble nature. Consequently, these materials persist, harming aquatic life and accumulating in soil and water systems. Aida’s team focused on solving this pressing issue with a new class of supramolecular plastics. Which dissolve into non-toxic, biodegradable components when exposed to seawater.
Creating Strong and Biodegradable Plastics
The researchers designed the plastics using two ionic monomers that form cross-linked salt bridges. These bridges provide strength and flexibility while maintaining biodegradability. One monomer, sodium hexametaphosphate, is a common food additive, and the other is a guanidinium ion-based compound. Bacteria metabolize both components, ensuring that the plastic decomposes fully.
Despite concerns that supramolecular plastics might be weak due to their reversible bonds, Aida’s team engineered materials that resist breaking down unless exposed to specific electrolytes, such as seawater salts. This selective reversibility is key to the material’s strength and degradability.
Manufacturing and Applications
The production process begins by mixing the two monomers in water, resulting in two distinct liquid layers. The researchers separated the viscous layer, which contains the salt-bridge structures, and dried it to create the final plastic. Without this desalting step, the material would crystallize and become brittle.
The resulting plastic exhibits properties comparable to or exceeding conventional plastics, including non-toxicity, non-flammability, and reshaping capabilities at high temperatures. By adjusting the monomer composition, the team created variations ranging from hard, scratch-resistant plastics to flexible, rubber-like materials. These adaptable properties make the plastic suitable for diverse applications, including 3D printing, medical uses, and weight-bearing structures.
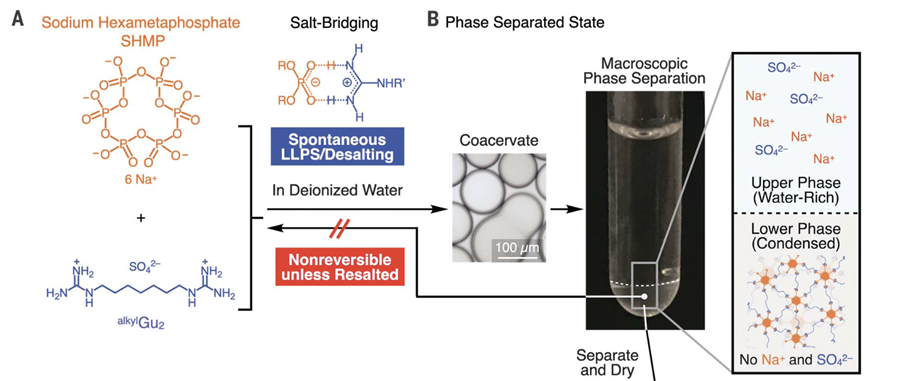
(A) Molecular structures of SHMP (orange) and a guanidinium sulfate (Gu)–based monomer. (B) Spontaneous liquid-liquid separation after mixing aqueous solutions of the monomers. The upper liquid is water-rich, containing sodium and sulphate ions, while the lower liquid is viscous and contains the 3D cross-linked supramolecular polymer. Courtesy of Bye-bye microplastics: new plastic is recyclable and fully ocean-degradable.
Recycling and Environmental Benefits
The new plastic also demonstrates impressive recyclability and environmental compatibility. Dissolving the material in seawater allowed researchers to recover over 90% of the sodium hexametaphosphate and 82% of the guanidinium for reuse. In soil, sheets of the plastic degraded within 10 days, enriching the soil with phosphorus and nitrogen, similar to a fertilizer.
A New Era of Sustainable Plastics
“This new material creates a family of plastics that are strong, stable, recyclable, and eco-friendly,” Aida explains. By avoiding microplastic generation and offering a versatile range of applications, this discovery marks a significant leap toward sustainable plastic solutions.
To read the study click here.

Really enjoyed this article. Excited to see how this progresses.