Leachables in Medical Plastics – What’s The Issue?
What are leachables in medical plastics? Why are they relevant and what is the issue? Diving into this topic impacts us all.
Plastic technologies have come a long way over the last 100 years. With historic developments in the world of polymers, developments such as sterility and efficacy, it is no surprise the medical industry relies heavily on plastic products. As of 2020, the global medical plastics market was worth $22.26 billion and growing. Yet, society still faces challenges with the safety of polymer-based products. Challenges like leachability impact us daily. Diving into what leachable compounds are, evaluating current technologies used to qualify and quantify leachables, and discussing today’s best practices in the medical field, helps to wrap our heads around this topic. Further, understanding the fundamentals and challenges facilitates scientific collaboration and the transport from problem to solution.
You Can Also Read: Healthcare Plastics Circularity
Leachables in Medical Plastics – What Does This Mean?
In its simplest form, leaching is the migratory process of chemical substances departing from a matrix and into the surrounding environment. With regards to plastics in the medical field, leachables are migrating chemical substances that move from a plastic material, such as medical packaging or medical devices, into a medical product or solution, such as pharmaceuticals, or even directly into humans. To break this down further, the leaching process can occur when a polymer comes into contact with water or other solvents or when a polymer is subject to specific conditions related to pressure, long-term use, or elevated temperatures. The polymer structure is also at play here – the porosity, crystallinity, and cross-linking within the polymer matrix can impact how easily substances migrate. Leachables can result from additives, the plastic itself, or contaminants derived during the manufacturing process. Plastic stabilizers, which prevent degradation, and additives, which modify properties, can leach out when exposed to solvents or as a result of an elongated shelf life. Further, the manufacturer’s choice of plastic materials and additives can influence the level and types of leachables. This leads manufacturers down the path of balancing performance and safety during product design. Thus, it is no surprise that sacrificing safety presents challenges.
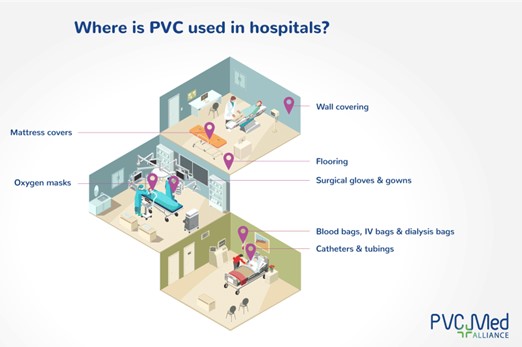
PVC in hospitals. Image courtesy of The European Council of Vinyl Manufacturers.
What Is The Issue With Leachables?
As you might have guessed, leachables in medical plastics can be problematic. Not only can a leachable migrate from the plastic packaging into the product during its normal shelf life, leachables can also migrate from products into humans via direct contact with bodily fluids, implantable devices, and drug delivery systems. As a result of these migratory tendencies, leachables in medical plastics come with their share of concerns:
- Safety. Leachables can lead to varying health risks including allergic reactions, toxicity, obesity, and carcinogenicity. Further, compounds like phthalates and Bisphenol A (BPA) can interfere with hormonal systems, leading to developmental, reproductive, and metabolic disorders. For example, phthalates such as DEHP are found in many polyvinyl chloride (PVC)-based medical devices and pose potential risks like endocrine disruption. Additionally, BPA, used to soften PVC, is also a known endocrine disruptor linked to developmental and reproductive issues.
- Efficacy. The presence of leachables may interfere with the effectiveness of pharmaceuticals or medical devices, potentially altering their intended performance. Further, leachables can compromise the mechanical or physical properties of the polymer. For example, if plasticizers leach out of medical tubing, the tubing can become stiff and prone to degradation. When additives leach out, the polymer can change, and the capacity to protect products like pharmaceuticals is compromised.
- Longevity and Stability. Over time, leachables can impact the stability of products, particularly in sterile environments or when stored for extended periods. For example, the presence of leachables can degrade a drug product, shortening its shelf life.
In consideration of these factors, testing and evaluation of leachables are critical in the use and advancement of medical plastics. Enter: qualification and quantification.
Qualifying and Quantifying Leachables
The good news is that thorough leachables qualification and quantification testing is conducted by manufacturers to ensure the safety of their products. This is especially true of applications that involve extensive contact with body tissues or fluids, such as IUDs, implantable drug delivery devices, long-term wound dressings, vascular grafts, stents, and pacemakers. Each of these devices must undergo testing that assesses for leachables. Analytical techniques for leachable detection include, but are not limited to, GC-MS, LC-MS, MS/MS, ICP-MS, FTIR, UV-Vis, and NMR. Typically, research and regulatory scientists utilize multiple techniques for a detailed evaluation. Despite these efforts, there are still challenges in leachables testing. Challenges like sensitivity enable the analysis of leachables at low concentration levels. Thus, Low Detection Limits Mass Spectrometry (LDL-MS) is essential for investigating leachables in medical materials and products.
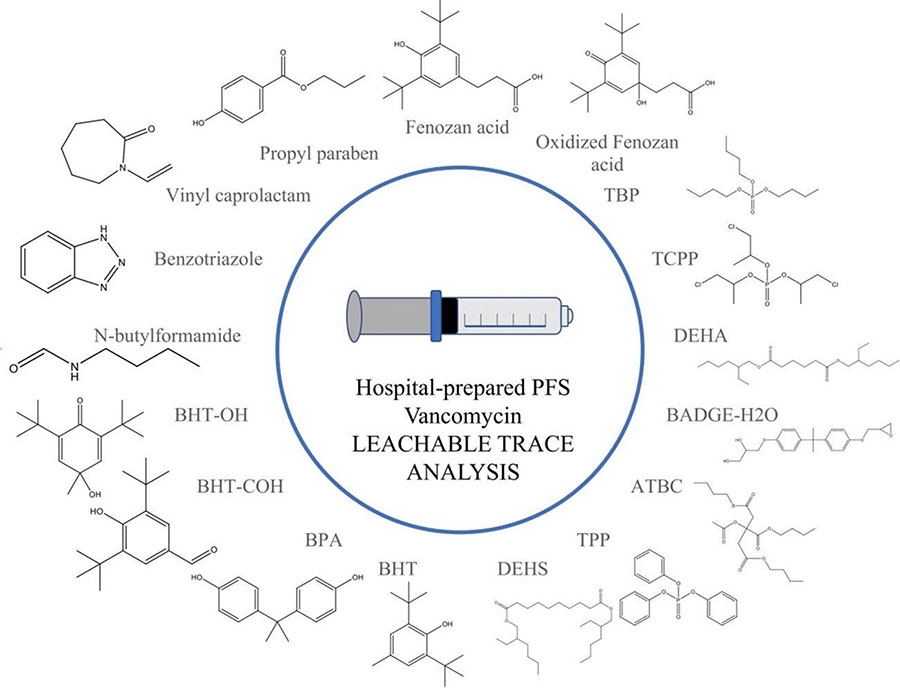
Seventeen plastic additives, formally identified, found in a hospital pharmacy-prepared prefilled vancomycin syringe for pediatric use. Image courtesy of Journal of Pharmaceutical and Biomedical Analysis.
Leachables Best Practices
U.S. regulatory agencies like the FDA require the pharmaceutical industry and other medical manufacturers to show harmful substances that leach out of their products. Detecting these compounds at trace levels requires high sensitivity and involves standardized regulatory protocols such as USP 1663, USP 1664, and ISO 10993-18. In addition to regulatory compliance, the medical industry is responsible for best practices. Best practices include material selection, comprehensive leachable and long-term stability studies, and risk assessment reports. These best practices can ensure product safety and help adhere to the regulatory expectations defined by the FDA. By following these best practices and investing in subsequent research, we can protect patient health and maintain product quality.
