Elemental Sulfur Turns Silicone into Repairable Vitrimer

Researchers from New Zealand developed a method to transform commercial-grade silicone into a repairable vitrimer by incorporating elemental sulfur.
Polysiloxanes, commonly known as silicones, have a highly cross-linked structure that makes them difficult to repair or recycle. As a result, industries generate significant silicone waste, contributing to environmental concerns. To address this, researchers from New Zealand have developed a repairable, commercial-grade silicone with dynamic S-S cross-links. This innovation offers a breakthrough solution for sustainability. It reduces silicone waste and provides a sustainable use for elemental sulfur, a byproduct of petroleum refining.
You can also read: Anticipating Change: The Move to Siloxane-Free PPAs
Transforming Commercial – Grade Silicone into Vitrimer
Researchers successfully modified the synthesis process of polysiloxanes by incorporating sulfur-based cross-links into commercial silicone. This resulted in vitrimers with self-healing and reprocessing capabilities. They interrupted the traditional platinum-catalyzed hydrosilylation reaction after two hours of mixing PDMS and PHMS at 25°C. At 180°C, they introduced sulfur, which halted hydrosilylation and initiated sulfur vulcanization. The outcome was stable polymers with enhanced hydrophobicity and self-healing properties.
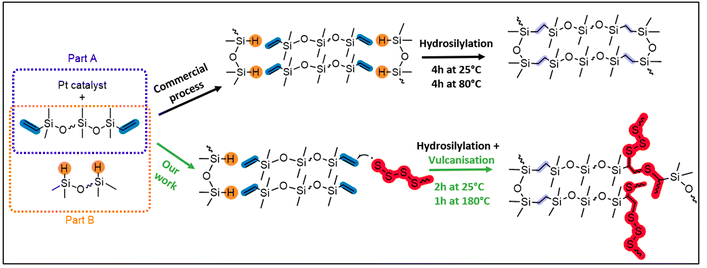
The image illustrates polysiloxane’s conventional synthesis (top route) utilizing parts A and B molding kits. It also shows the modified synthesis (bottom route) that employs S8 to produce X-poly(siloxane-r-S) with self-healing properties. Courtesy of Converting Commercial-Grade Silicone into a Vitrimer using Elemental Sulfur.
Enhancing Hydrophobicity
Incorporating sulfur into polysiloxane increased its hydrophobicity, particularly at higher sulfur levels. A 0-poly (siloxane-r-S) had a contact angle of 105.91º, while a 15-poly (siloxane-r-S) measured 111.4º, reflecting a 4.93% increase. Despite this, sulfur incorporation did not significantly affect solubility or swelling behaviors, preserving the material’s stability.
Boosting Self-Healing Properties
Moreover, the incorporation of sulfur significantly enhanced the material’s self-healing capabilities. Samples with 5% and 10% sulfur were fully repaired after 30 and 24 hours, respectively. Dynamic S-S bonds facilitated this recovery, maintaining structural integrity and elasticity after each repair. These improvements highlight the potential of sulfur-modified silicones for applications requiring durability and resilience.
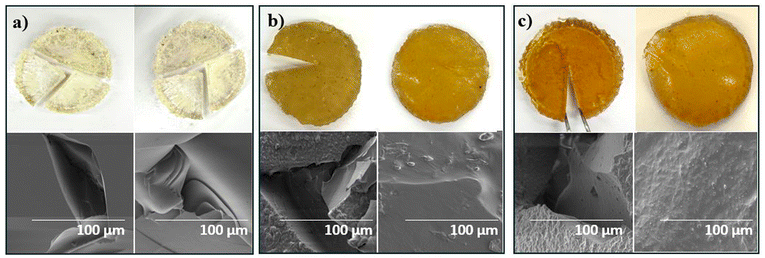
The image displays the thermally repairable properties of polysiloxane samples with different weight percentages of sulfur (X), along with their corresponding SEM images. The images show the samples before (left) and after (right) the healing process for X-poly(siloxane-r-S), where X represents (a) 0, (b) 5, and (c) 10. Courtesy of Converting Commercial-Grade Silicone into a Vitrimer using Elemental Sulfur.
Evaluating Thermal Stability
In addition to enhancing mechanical properties, higher sulfur content slightly reduced thermal stability. Thermogravimetric analysis showed lower temperatures for weight loss with increasing sulfur levels. However, S-S cross-links introduced unique thermal behaviors like depolymerization and reprocessing, making these materials ideal for dynamic bonding applications.
Paving the Way for Sustainable Innovations
In summary, by incorporating sulfide cross-links into silicones, researchers have created vitrimers with enhanced reparability and recyclability. This breakthrough offers environmental and economic benefits by reducing waste and utilizing sulfur, a petroleum byproduct. It opens new doors for self-healing materials in industries such as aerospace, automotive, and electronics, contributing to a more sustainable future.
You can read the full article here.
