Cleaner Latex, Safer Planet: Ammonia-Free Solutions

The rubber industry employs ammonia to preserve latex by preventing bacterial growth and stabilizing particles. However, ammonia poses significant health risks, including severe skin burns, eye damage, and respiratory issues.
Natural rubber latex is a complex mixture of polyisoprene rubber, proteins, sugars, amino acids, lipids, and minerals when extracted. The latex is highly susceptible to bacterial decomposition and coagulation upon contact with air. Historically, researchers used ammonia, tetramethyl thiuram disulfide (TMTD), and zinc oxide (ZnO) to prevent this. These chemicals, however, have adverse effects on both the environment and the health of workers in rubber plantations and industries.
Innovative Methods for Eco-Friendly Acidic Latex Preservation
Researchers from the Polymer Engineering Center developed two innovative methods for preserving and stabilizing natural rubber latex in an acidic environment, eliminating the need for ammonia and other dangerous chemicals.
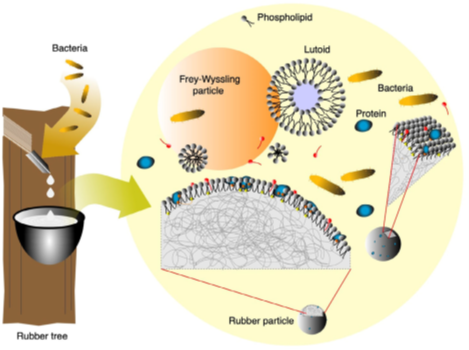
Rubber latex architecture before the putrefaction and coagulation process. Courtesy from: Environmentally safe preservation and stabilization of natural rubber latex in an acidic environment. SPE Polym. 2023
Dodecyl Benzene Sulfonic Acid (DBS)
The first method involves dodecyl benzene sulfonic acid (DBS) to stabilize and preserve the latex. A 2% volume of DBS added to the latex creates an acidic environment with a pH between 3.5 and 4.2.
This method not only preserves the latex for up to four years but also results in rubber with superior mechanical properties. In the rubber industry, maintaining alkaline conditions is typical to prevent coagulation, so the acidic environment is counterintuitive.
Researchers hypothesize that DBS reacts with glutathione in the latex to form glutathione dodecylbenzene sulfonate (GDBS). This compound acts as both a surfactant and an antibiotic, effectively stabilizing the latex and killing bacteria.
Ethoxylated Tridecyl Alcohol (ETA) and Hydrofluoric Acid
The second method uses tridecyl alcohol ethoxylated for stabilization and hydrofluoric acid for preservation. Adding 1% by volume of ethoxylated tridecyl alcohol stabilizes the colloidal suspension, while 0.4% by volume of a 50/50 hydrofluoric acid/water solution preserves the latex. Researchers believe that DBS reacts with glutathione in the latex, forming glutathione dodecylbenzene sulfonate (GDBS).
This compound serves as both a surfactant and an antibiotic, effectively stabilizing the latex and killing bacteria. This method also extends the shelf life of the latex and maintains its stability.
Enhanced Mechanical Properties with Ammonia-Free Stabilization
Mechanical tests comparing ammonia-free and ammoniated natural rubber materials revealed significant findings. Initial stress measurements using a dynamic mechanical analyzer (DMA) showed that ammonia-free stabilized and preserved rubber exhibited initial stress 2.5 times higher than its ammoniated counterpart. Additionally, rubber stabilized with ETA and preserved with hydrofluoric acid demonstrated initial stress twice that of ammoniated rubber, indicating the presence of chain extenders absent in ammonia-stabilized rubber.
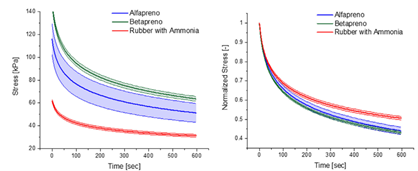
Using the NETZSCH Gabo Eplexor 500N dynamic mechanical analyzer, researchers imposed a 30% tensile strain on a rectangular specimen to obtain results from the relaxation tests. Courtesy of Environmentally safe preservation and stabilization of natural rubber latex in an acidic environment. SPE Polym. 2023
Furthermore, large amplitude oscillatory shear strain tests indicated higher stress levels in ammonia-free samples, especially those stabilized with environmentally friendly methods like ETA and hydrofluoric acid. Despite these variations in stress levels, the overall viscoelastic behavior of ammonia-free latex remained comparable to that of ammoniated latex.
Therefore, ammonia-free stabilization and preservation techniques not only enhance mechanical properties but also offer environmental benefits without compromising material performance.
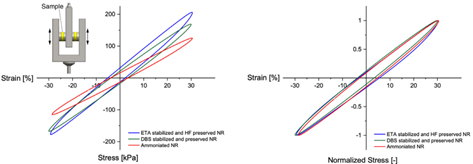
Researchers used the NETZSCH Gabo Eplexor 500N dynamic mechanical analyzer to conduct cyclical testing on two cylindrical samples in double shear. They imposed a 30% dynamic strain at 1 Hz and 21°C to obtain results from the dynamic tests. Courtesy from: Environmentally safe preservation and stabilization of natural rubber latex in an acidic environment. SPE Polym. 2023
The development of environmentally safe methods for the preservation and stabilization of natural rubber latex marks a significant advancement in the rubber industry. By eliminating the use of hazardous chemicals like ammonia, these new techniques not only protect the environment and improve worker safety but also produce superior rubber products.
These methods have the potential to revolutionize natural rubber production, aligning it with sustainable and health-conscious practices.
To read more: Environmentally safe preservation and stabilization of natural rubber latex in an acidic environment.
