Nanotechnology: Advancing the Degradation of Plastics
Nanotechnology offers techniques for degrading plastics that help address pollution issues caused by improper disposal.
The widespread use of various polymers in multiple industries is primarily due to their durability and high stability, which ensure resistance to extreme conditions, including temperatures, moisture, and UV exposure. However, this very trait also contributes to the primary environmental concern posed by plastics. Ultraviolet light is required to break down the molecules of plastics spontaneously into progressively smaller pieces that microbes can metabolize and fully degrade. Typically, degradation times for plastic bags falls between one of two ranges: 10–20 years or 500–1000 years. Similarly, estimates for “plastic” bottles suggest decomposition times ranging from over 70 to 450 years.
You can also read: Antimicrobial Nanofilm Pioneering Food Preservation
The Role of Nanotechnology in Plastics Degradation
Mechanical recycling of plastics is an essential tool in a sustainable economy. However, this process continues to encounter challenges that impedes its global effectiveness. Despite the presence of a strong infrastructure and recycling facilities, limitations such as the scarcity of collection and sorting sites, complexities in segregating plastic types and the elevated costs of gathering and treating plastic waste persist. Nanotechnology emerges as a modern solution to the challenge of plastics accumulation in the environment. Inorganic fillers can be added to polymers to form nanocomposites to enhance its mechanical and thermal properties and degradability. Nanocomposites are polymers with dispersed inorganic fillers in a polymeric matrix. The incorporation of nanoparticles such as TiO2 into polymers has proven to increase the composites degradability.
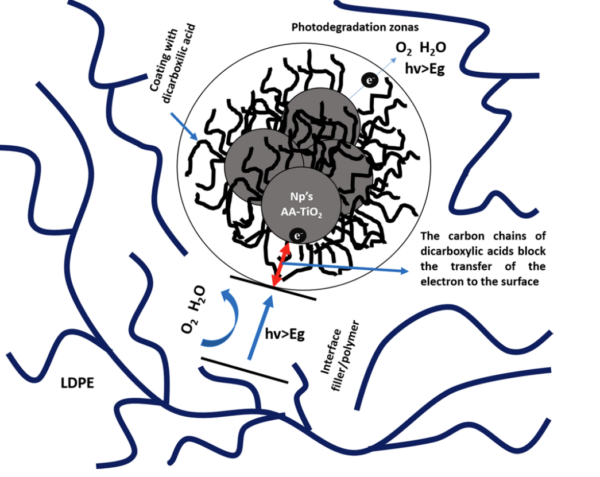
Representative scheme of the overall interaction between particles and polymer chains by effect of the coating. Courtesy of Chemical modification of titanium dioxide nanoparticles with dicarboxylic acids to mediate the UV degradation in polyethylene films.
A Successful Case of Degradation of PE Films
In the scientific study “Enhanced photocatalytic degradation of PE film by anatase/γ-MnO2” https://www.sciencedirect.com/science/article/pii/S0141391023000472 researchers evaluated the photocatalytic effects of TiO2 and MnO2 on PE degradation. In the figure, it can be evidenced how the use of nanocomposites promotes a greater loss of mass in the degradation of the polyethylene film when subjected to 90 hours of UV radiation. Even the combination of two different materials further favors this mass loss and photocatalytic activity.
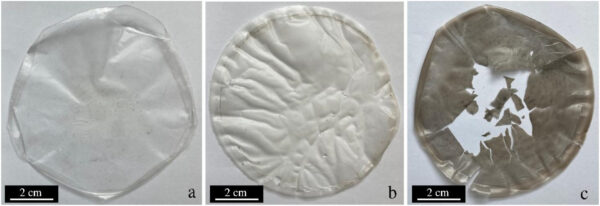
Images of PE (a), PE/TiO2 (b), PE/TiO2&MnO2 (c) films after 90 h of UV-irradiation. Courtesy of Enhanced photocatalytic degradation of PE film by anatase/γ-MnO2.
A Step Towards Sustainable Plastic Management
As the demand for sustainable solutions to reduce plastic pollution continues to rise, integrating nanotechnology into polymer manufacturing emerges as a promising alternative for future industrial applications. This innovation holds the potential to enhance the recyclability of plastics, aligning them more closely with existing methods such as thermal degradation, landfilling, and incineration. However, while nanoparticle modification offers a step forward, it alone cannot serve as a comprehensive solution to the complex issues regarding plastic waste. It is imperative to maintain a multifaceted approach, emphasizing the reduction, reuse, and recycling of plastics. This needs not only individual actions but also the reinforcement of waste management systems, encompassing improved collection, sorting, recycling, and responsible disposal practices. Achieving sustainability in the realm of plastics demands collaborative efforts from all stakeholders, from producers to end users. It is a shared responsibility that requires collective action and commitment to enact meaningful change.

We have studied the effect of anatase on polyolefins photodegradation in the past. From our previous results,I feel that the use of anatase TiO2 will fragment the polymer and not reduce its molecular weight sufficiently to make it biodegradable. This nanocomposite will only contribute to increase the amount of microplastics in the environment.
Hello Marco! If your work has been published in a scientific journal, we can review it and write about it! Regards