TFA: A Sustainable Alternative to PFAS?

Trifluoroacetic acid (TFA) stands out as a promising alternative to per- and polyfluoroalkyl substances (PFAS), known as “forever chemicals,” due to its lower environmental impact while maintaining similar benefits.
The increasing search for sustainable and eco-friendly chemical solutions in manufacturing and product development has become more critical amid rising concerns about the environmental and health implications of PFAS usage. These substances, notorious for their water-repellent and non-stick qualities, pose significant environmental and health risks because of their durability and tendency to accumulate.
You can also read: Fluoropolymers Life Cycle and PFAS Contamination
Understanding TFA
Unlike PFAS, which are resistant to degradation due to their strong carbon-fluorine bonds, TFA is a simpler compound. With three fluorine atoms on a two-carbon chain, TFA excels in dissolving organic compounds and shows promise in various applications. Its simpler structure allows for easier degradation under certain environmental conditions, making it a less harmful option.
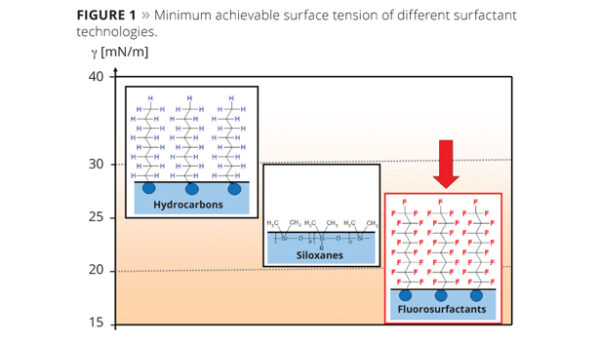
Surface tension of different surfactant technologies. Courtesy of A New Generation of High-Speed Fluorosurfactants.
A PFAS Replacement
Various industries are now investigating Trifluoroacetic acid as a substitute for PFAS, particularly in textiles, cookware, and firefighting foams. Researchers are focusing on TFA-based compounds for textiles to achieve water repellency without the persistent environmental impact typical of PFAS. Similarly, the cookware industry is exploring TFA as an additive in non-stick coatings, aiming to preserve cookware’s non-stick features without the associated long-term risks.
In firefighting, TFA-based foams are under development as safer alternatives to traditional PFAS-containing foams. These foams are known for their effectiveness against fuel fires but contribute significantly to environmental pollution. TFA’s introduction promises effective fire suppression capabilities with a reduced environmental footprint.
However, the effectiveness of TFA and similar alternatives often hinges on their surface tension properties. For example, long-chain fluorosurfactants based on C8 achieve a low surface tension due to their dense hydrophobic group packing. Conversely, short-chain surfactants, lacking in hydrophobicity, cannot compact as tightly, leading to higher surface tensions that may not meet specific application needs.
Environmental and Health Considerations
Despite TFA’s potential, its environmental and health effects warrant thorough examination. Preliminary studies indicate that TFA is less prone to bioaccumulation and has a shorter environmental persistence than its long-chain counterparts. Nevertheless, detailed studies are essential to evaluate its toxicity, biodegradability, and overall environmental impact. Current research suggests that most short-chain PFASs, including TFA, do not bioaccumulate in food chains and are rapidly excreted from humans, although some reports state that Trifluoroacetic acid has the potential to bioaccumulate in plant material.
But, even in the absence of evidence that TFA presents a significant risk to humans or the environment, its persistence due to its environmental stability, combined with constant or increasing emissions, will likely result in significant accumulation in the environment. TFA contamination could become widespread, persistent, and hard to eliminate. Discovering any harmful effects of TFA in future studies would pose a major challenge. Already, significant and rising levels of TFA appear in surface water, rain, fog, sewage plants, snow, air, and sediments.
![Surface distribution plot depicting the multiplicative increase of TFA distributions predicted by changeover from HFC use to HFO use as described by Holland et al. [57]. Reproduced with permission from authors. American Chemical Society.](https://www.plasticsengineering.org/wp-content/uploads/2024/03/sustainability-16-02382-g003-600x527.png)
Surface distribution plot depicting the multiplicative increase of TFA distributions predicted by changeover from HFC use to HFO use as described by Holland et al. Courtesy of Trifluoroacetic Acid: Toxicity, Sources, Sinks and Future Prospects.
Moving from PFAS to TFA marks a major step in reducing risks from “forever chemicals.” Trifluoroacetic acid offers hope with less lasting environmental impact. Yet, the scientific community must continue researching and monitoring its use closely. As we focus more on sustainability, creating safer chemicals becomes key for both the environment and our health. Also, some brands aim to avoid all fluorinated options, impacting TFA’s market and availability.

TFA has been found in water sources worldwide, including the US, where a sixfold increase in TFA levels over 23 years has been reported. In Germany, TFA concentrations in rainwater have surged fivefold in two decades. The UK has yet to set limits for TFA in drinking water, despite being identified as a substance of concern. Across Europe, TFA has been detected in alarming concentrations, with 79% of samples exceeding the EU Drinking Water Directive limit for total PFAS.
https://tappwater.co/blogs/blog/pfas-trifluoroacetic-acid-tfa-how-to-remove-filter-tfa-from-tap-water
Hello Jay! Thank you very much for your comment. The topic of discussion about TFA in this article focuses on its bioaccumulation rather than its concentration. TFA is a simpler compound unlike other PFAS, which are resistant to degradation (C6, C8) due to their strong carbon-fluorine bonds. “Preliminary studies indicate that TFA is less prone to bioaccumulation and has a shorter environmental persistence than its long-chain counterparts. Nevertheless, detailed studies are essential to evaluate its toxicity, biodegradability, and overall environmental impact,” as mentioned in the article. You can also read the study Trifluoroacetic Acid: Toxicity, Sources, Sinks and Future Prospects, which the article is based on.